Treatment platform
with Dry Eye Disease as
primary target ¹
1) Investigational treatment systems, Abilion currently has no approved products.
A potential treatment adressing a large unmet need
What is DED?
Dry Eye Disease (DED) is an inflammatory disease with symptoms of irritation, redness, discharge, and easily fatigued eyes. The symptoms range from mild and occasional to severe and continuous. Eye dryness is also one of the leading causes of discontinuation of contact lens use.
“More than 30 million people in the United States have DED, which may be the single greatest opportunity in medical eye care.”
– Karpecki A, Review of Optometry, May 2019
A treatment based on the INMEST Technology
Intra-Nasal MEchanical STimulation (or INMEST for short) is a novel treatment approach pioneered by prof Jan-Erik Juto during his career as a Medical Doctor and Ear-Nose-Throat (ENT) Surgeon at the Karolinska University Hospital in Stockholm, Sweden. INMEST is delivered using a soft and pliable catheter that is inserted into the nasal cavity where it gently stimulates the deeply innervated tissues that line part of the cavity.
INMEST technology is being investigated in clinical trials.
Abilion’s medical device is protected by granted patents and pending patents.
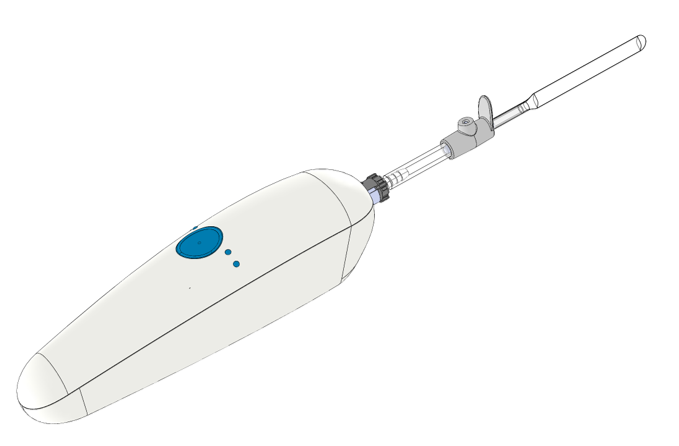
A product system for self-treatment at home
Handheld treatment device
Built for self-administered home treatment and designed to be as convenient and easy to use as possible (patents pending).
The device consists of a controller and a disposable catheter.
Mobile app
The mobile app connects to the handheld controller and permits the patient to track symptoms over time. Usage patterns and symptom can then be shared with a clinician if the patient needs or desires professional guidance.
Cloud-based treatment management system
The cloud solution gives the clinician a clear view of the patient’s usage and symptoms development, providing valuable supporting data for guidance and recommendations.
